Method of atomic emission spectral analysis allows to determine the chemical composition of the substance. Analyzed material is dispersed under the influence of the excitation source (electric arc or spark, flame, plasma). For a short (about 10-7c) interval electrons of atoms or ions of the vaporized material are moving to higher energy levels and return to sustainable unexcited state. This energy is released as radiation at a particular wavelength.
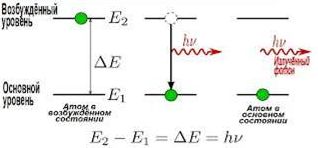
The result is a series of spectral lines, specific to a particular chemical element, which may be registered with optical instruments. Wavelength detected in the analysis of the atomic ranges from 150 to 800 nm.

Spectrographic analysis is divided on:
- visual spectral analysis (visible range 400-760 nm);
- spectrographic method (registration of atomic spectra on a photographic plate);
- spectrometric method (instruments with photoelectric recording of the spectrum).
Technique of modern atomic spectral analysis of steels and alloys often uses ultraviolet part of the spectrum. As a means of recording are used CCD sensors (Charge-Coupled Device).
